 Science (2025). DOI: 10.1126/science.ado5068″
Science (2025). DOI: 10.1126/science.ado5068″width=”800″ height=”530″/>
A team of researchers from Stanford University has uncovered crucial insights into a fundamental question of life: how enzymes accelerate vital biochemical reactions exponentially. Utilizing over 1,000 X-ray images capturing the dynamic behavior of enzymes, their discoveries could revolutionize various fields, from foundational scientific research to pharmaceutical development. This research may also inspire a re-evaluation of how enzymatic processes are taught in educational institutions.
“When I say enzymes can dramatically speed up reactions, it’s incredible—they can be a trillion trillion times faster for certain processes,” remarked Dan Herschlag, the lead researcher and a professor of biochemistry in the School of Medicine. “Enzymes function as astonishingly efficient machines, yet our grasp of their operational mechanics has been quite limited.”
According to Herschlag, while several theories exist, biochemists have struggled to convert these theories into a precise understanding of the interactions—both chemical and physical—that enable these swift reaction rates. This gap in knowledge has hindered their ability to predict enzyme activity or to create novel enzymes as effectively as those found in nature, which would have significant implications across various industries and in medicine.
“With our detailed ensemble of enzyme states, we’ve successfully quantified and precisely articulated the attributes of enzymes that contribute to their catalytic activity,” explained Siyuan Du, the study’s first author and a doctoral student in Herschlag’s lab. Their research, featured in the February 14 edition of Science, marks a significant step forward in the field.
“This new methodology—and our understanding—sets the stage for designing enzymes that could match those found in nature. However, this is only the beginning, and further research is required to reach that aspiration,” Herschlag emphasized.
Unraveling Enzymatic Mysteries
Prior to 1926, biochemists were bewildered by reactions exclusive to living organisms, attributing them to a perplexing “vital force.” This changed when James Sumner isolated urease, the first enzyme, in a groundbreaking study that earned him a Nobel Prize. In the years that followed, biochemists have sought to uncover how enzymes perform reactions with such speed and specificity. Although they can explain these processes conceptually, a quantitative understanding has remained elusive, fueling ongoing debates within the scientific community.
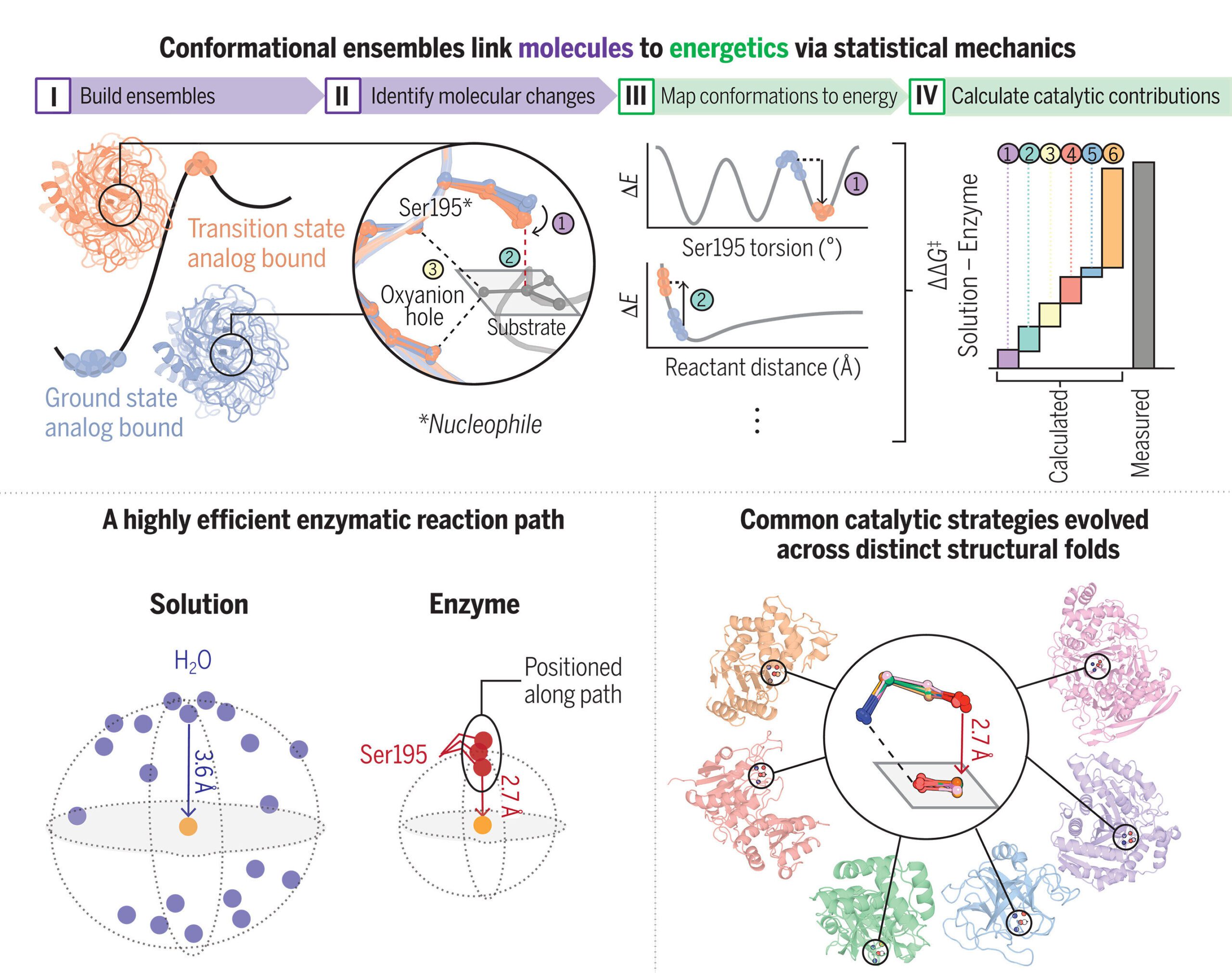
Du and Herschlag expanded on the prevalent biophysical perspective that enzymes are dynamic rather than static structures. Their focus lies on “ensembles,” illustrating how enzymes transition between various physical states—or conformational ensembles—during catalysis.
“Current models largely rely on specific arrangements of reacting molecules on the enzyme’s surface to facilitate reactions,” explained Herschlag. “However, the importance of positioning has been a topic of contention, largely due to the absence of a measurable framework until now with our novel ensemble-based approach.”
“Enzymes are constantly in flux, existing in an ensemble of states, and the reaction rate is determined by probability distributions within that ensemble,” elaborated Du.
In their study, the researchers focused on serine proteases—a family of enzymes commonly highlighted in biochemistry textbooks to illustrate enzymatic behavior.
By examining these ensembles and contrasting the reaction states of the enzymes with those of non-catalyzed reactions in pristine water,Du and Herschlag dissected the individual energy contributions exactly at the active site—the critical juncture where enzyme and substrate interact—to reveal their chemical and physical roles in enhancing reaction rates.

Harnessing Potential Energy
For example, Du observed that in the active site, an oxygen atom from an enzyme moves closer to a carbon atom of its target molecule, akin to the behavior of a coiled spring. However, she warned against overly simplistic views, noting that the movements of individual atoms are vastly different from the smooth action of a spring.
“There’s a certain tension drawing these atoms together, and when the reaction occurs, that accumulated energy drives the reaction forward, resulting in a much quicker process,” she explained. Du noted that these catalytic behaviors were common across all serine proteases, as well as in over 100 additional enzyme families.
“The natural world has independently evolved these catalytic mechanisms across various enzyme families. This signifies that these are not isolated phenomena; instead, they represent evolutionary strategies discovered repeatedly in nature. This insight provides an opportunity for us to mimic nature, leveraging these characteristics to design and construct new enzymes,” Du added.
Regarding future directions, Herschlag and Du assert that articulating the extraordinary capabilities of these essential biochemicals in straightforward chemical terms could transform biochemistry education and accelerate advancements in numerous significant arenas.
“In summary,” Herschlag said, “Gaining a more profound understanding of enzymes is crucial if we aim to effectively manipulate them and engineer superior alternatives.”
For more details: Siyuan Du et al, “Conformational ensembles reveal the origins of serine protease catalysis,” Science (2025). DOI: 10.1126/science.ado5068
Provided by Stanford University
Citation: Revolutionary insights into enzymes’ power may alter biochemistry (2025, February 14) retrieved 15 February 2025 from https://phys.org/news/2025-02-power-enzymes-reshape-biochemistry.html
This document is copyright-protected. Apart from fair use for personal research or study, no part may be reproduced without written consent. The information provided is for informational purposes only.