Investigation and Results
Case Investigation
Medical providers from SMC Health conducted interviews with the traveler, revealing that prior to his departure to Africa from the United States, he had noticed a minor “ingrown hair” in the pubic area. This condition worsened during his one-week stay in East Africa, becoming increasingly tender and swollen. Upon his return to the U.S. on day 0, he experienced a perceived fever during his flight (Figure 1). By day 2, he sought evaluation at two urgent care centers due to the progression of symptoms, including fever, shortness of breath, extreme fatigue, and nausea, alongside new unilateral inguinal adenopathy. Examination revealed a 1 cm purulent ulcer at the base of the penis and multiple tender lymph nodes in the left groin area, while no other rashes or lesions were identified. Unfortunately, his travel history was not obtained during these evaluations. Tests for various infectious diseases returned negative results, leading to a prescription for oral trimethoprim-sulfamethoxazole and amoxicillin-clavulanic acid for suspected pubic folliculitis.
On day 4, he revisited an emergency department (ED) reporting continuing fever, chills, and headaches. Though the pubic ulcer appeared to be healing, lymphadenopathy persisted, along with a new small pustule on his right forearm. Laboratory assessments indicated high levels of erythrocyte sedimentation rate and C-reactive protein, signaling inflammation, along with an unusually high creatinine level. Consequently, he was admitted for presumed inguinal cellulitis that had not responded to oral antibiotics and acute kidney injury.
While receiving intravenous (IV) vancomycin in the ER, he experienced flushing and a non-itchy rash on his right arm, which subsided when the infusion rate was adjusted. Additionally, broad-spectrum IV antibiotics were administered as a precaution. Over the next few days, he developed a pruritic rash described as “tiny bumps” on his arms, chest, and abdomen; initially believed to be a reaction to medication, this rash improved with topical 2.5% hydrocortisone cream. By day 6, after a notable improvement in his condition and normalization of his kidney function, he was discharged on a regimen of oral doxycycline and cefpodoxime.
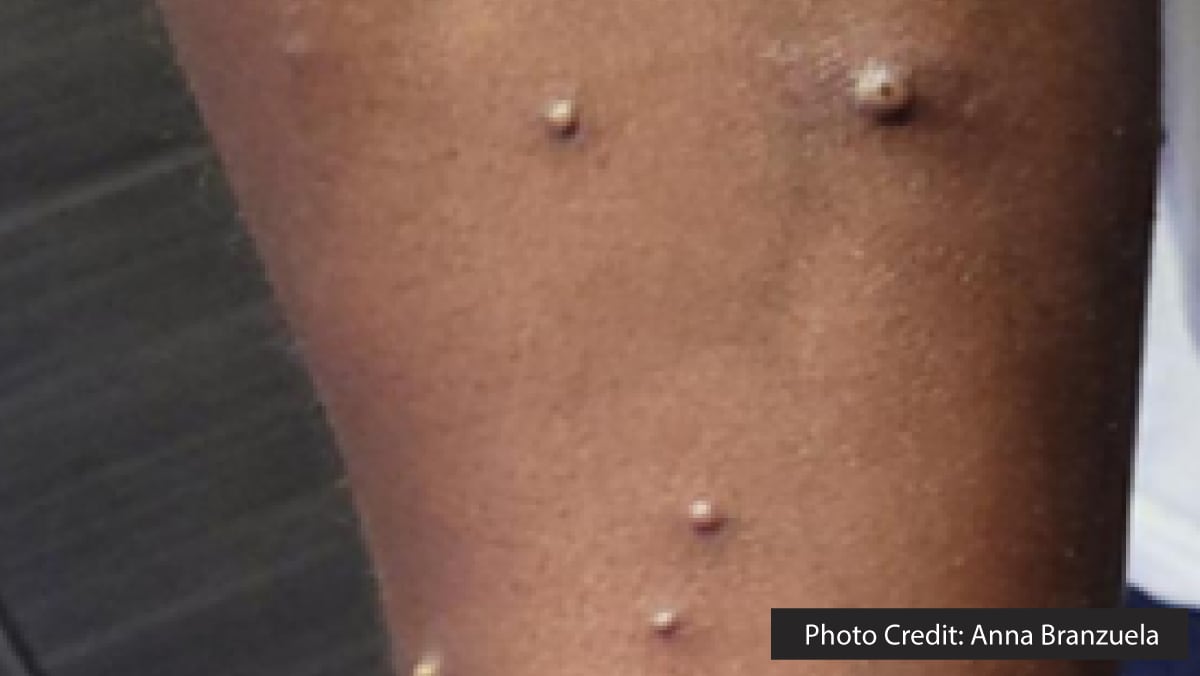
The next day, however, he experienced worsening inguinal pain, and new discrete papular, vesicular, and pustular lesions emerged on his face, back, arms, and legs (Figure 2), prompting yet another visit to the ED. It was during this consultation, one week after returning to the U.S., that his travel to East Africa was first discussed. A mild leukocytosis was noted, and a computed tomography (CT) scan indicated continued inguinal lymphadenopathy, ruling out loculated fluid collections. The ulcer at the base of the penis was dry with mild surrounding induration and no drainage. Pustules on his arm and back were swabbed and sent for testing for varicella zoster virus (VZV) and MPXV.
By day 11, the laboratory report confirmed the presence of NVO DNA (Ct = 25 [reactive]) and the absence of clade II MPXV DNA, pointing to a potential clade I MPXV infection. VZV tests returned negative. Swabs taken from the patient’s arm and leg were retested on day 11 by SMC Health, and subsequent analysis by CDPH VRDL the following morning confirmed the presence of clade I MPXV DNA (with diagnostic panMPXV positive, but clade Ia and clade II negative). On day 14, whole genome sequencing from day 11 specimens conducted at CDPH VRDL and CDC validated the strain as clade Ib MPXV.†
The patient noted attending social events and receiving a full-body massage during his travels in East Africa, reporting no sexual encounters during this time. He isolated at home until all lesions had healed, a status verified by a clinical examination from the local health department physician on day 23.
Contact Identification
A total of 83 individuals were identified as contacts and categorized into various risk groups§ (9): one high-risk household contact, four travel companions (categorized as uncertain or minimal risk), 10 flight contacts seated within 6 feet of the patient (also uncertain or minimal risk), and 68 health care personnel (HCP), of which three were deemed intermediate risk, 56 uncertain or minimal, and nine with no identifiable risk. Most of the contacts (77) were enrolled in a twice-daily automated symptom monitoring program for 21 days; six flight contacts were unreachable. Six HCP reported incidental lesions on their necks, backs, or legs; however, these lesions did not conform to typical mpox characteristics, and no orthopoxvirus DNA was detected in samples collected from these lesions. The household contact and four travel companions received JYNNEOS post-exposure prophylaxis, and no secondary cases were identified.
Public Health Response
Prior to the identification of the first clade I (specifically clade Ib) MPXV infection in the Americas, the CDC and its public health partners had already begun modifying clade II MPXV surveillance efforts to enhance readiness for clade I MPXV threats. Initiatives included the use of commercial and public health lab testing as a means of surveillance for clade I (i.e., NVO positive, clade II negative), the rollout of additional laboratory-developed tests in public health labs and the CDC (including panMPXV and clade I MPXV PCR assays at CDPH VRDL), and the creation and dissemination of public health and clinical guidance via CDC Health Action Network (HAN) and California Health Action Network (CAHAN) publications, alongside communication with the laboratory community through CDC Laboratory Outreach Communication Systems (LOCS). Furthermore, upon the international recognition and reporting of the clade I MPXV outbreak in Africa, CDPH established a statewide surveillance system to diligently monitor ELR data daily for NVO/clade II MPXV PCR results that could indicate a clade I MPXV infection (i.e., detection of non-variola orthopoxvirus DNA, with clade II MPXV DNA undetected). Following the discovery of this clade Ib MPXV case, public health interventions were swiftly implemented, including ongoing daily monitoring for any further suspicious ELRs during contact tracing, effective communication and guidance through HAN and CAHAN reports, and continued dialogue with local health departments and clinicians since this initial identification (6).









