A revolutionary cancer “vaccine” developed by researchers from Yale University has successfully eradicated the disease in nine patients.
Participants in the study, who were enrolled between March 2019 and September 2021, showed no signs of kidney cancer during a three-year follow-up in July 2023, signifying a significant achievement in cancer treatment.
The patients were suffering from stages three and four clear cell renal cell carcinoma (ccRCC), a type of kidney cancer that boasts a survival rate of only 10 to 15 percent over five years, killing 85 to 90 percent of those affected.
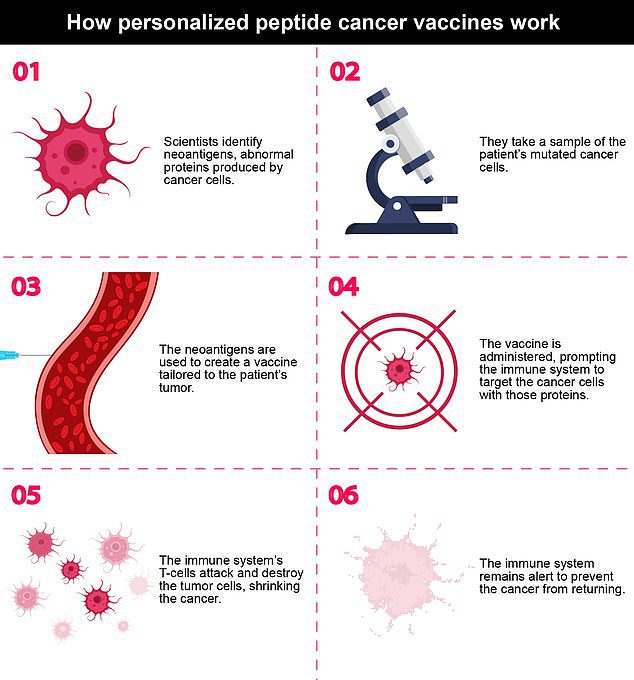
This innovative vaccine effectively targeted and eliminated remaining cancer cells post-surgery, while skillfully avoiding damage to healthy cells by being meticulously tailored to the individual biology of each patient.
Developed by a collaborative team at the Yale Cancer Center and Dana-Farber Cancer Institute, the vaccine aims to retrain the immune system to detect specific mutations present in a patient’s tumor that healthy cells do not possess.
Dr. David Braun, the primary author of the study and lead investigator at the Yale Cancer Center, emphasized, “The concept behind this trial was to accurately direct the immune system towards a target unique to the tumor.”
Cancer cases are not one-size-fits-all, and researchers are focused on developing personalized vaccines that can precisely eliminate cancer cells while also preventing recurrences, which affects 20 to 50 percent of patients.
While the primary focus of this research is on ccRCC, the outcomes could potentially influence treatment strategies across a range of medical disciplines if future studies confirm the vaccine’s efficacy.

The initial phase of the trial aimed to assess the safety of the vaccine and measure how well patients tolerated it.
At the conclusion of the study three years later, all nine participants who received the vaccine were cancer-free.
Kidney cancer ranks as the seventh most prevalent cancer among men in the United States and the tenth among women, with around 10 to 15 percent of stage IV ccRCC patients surviving beyond five years.
The recent study saw all nine participants receiving a total of seven doses of the vaccine — five during the initial phase and two as a booster.
Among the patients, four solely received the vaccine while five were also administered small doses of the immunotherapy drug ipilimumab to assess the vaccine’s performance independent of immunotherapy.
Administering lower doses of ipilimumab allowed researchers to determine if this immunotherapy could enhance the vaccine’s effectiveness.
Of the participants, seven had stage three disease, while two were diagnosed with stage four.
Every patient exhibited an immune response post-vaccination, suggesting the treatment successfully stimulated the body’s immune defenses. The immune system was able to recognize and respond to approximately 65 percent of the cancer-causing mutations in their tumors.
The vaccine employs small protein fragments designed to mimic cancer-related proteins, termed neoantigens, which assist the immune system in identifying and attacking the cancer cells.

Dr. David Braun, the lead investigator from Yale Cancer Center, communicated that the trial sought to guide the immune system to focus on a target particular to the tumor, aiming to minimize the risk of cancer recurrence for patients with advanced ccRCC.

Kidney and renal pelvis cancer affects 17.2 new cases and 3.4 deaths per 100,000 people annually. About 1.8 percent of people will be diagnosed with this cancer in their lifetime.

The vaccines, designed by Yale Cancer Center and Dana-Farber Cancer Institute, are aimed at retraining the immune system to recognize only the specific mutations present in a patient’s tumor that normal, healthy cells do not have.
Researchers analyzed the genetic profiles of each patient’s tumors to isolate their unique proteins, synthesizing peptides in the lab for identification. These peptides were then combined to formulate the vaccine.
Both groups — those receiving immune treatment and those who did not — yielded similar positive outcomes, and none of the participants reported severe side effects aside from mild flu-like symptoms post-vaccination.
Dr. Braun noted, “This robust and sustained activation of T cells was promising, indicating that we can generate a durable anti-cancer immune response with this vaccine.”
These findings were published in the journal Nature.
Further trials with a larger patient cohort are essential to accurately evaluate the vaccine’s effectiveness. Ongoing phase two trials will assess the combined impact of a similar personalized cancer vaccine (PCV) alongside the targeted therapy, Keytruda (pembrolizumab).
Although these initial results are not definitive, they offer a refreshing perspective in the field of oncology research, which has largely faced challenges in developing a versatile cancer vaccine that can effectively target a broad spectrum of cancers without allowing them to escape the immune response.
Renal cell carcinoma is ranked among the ten most prevalent cancers globally, with over 400,000 new diagnoses each year and approximately 80,000 new cases in the United States. The disease is more frequently diagnosed in men and has notably higher occurrences in Western countries.
Factors such as age, smoking, obesity, elevated blood pressure, genetic predispositions, and family history of kidney cancer can increase the risk of developing this type of cancer.
The rate of kidney cancer diagnoses has seen a steady increase, from 6.82 new cases per 100,000 in 1975 to 15.75 in 2022, potentially attributed to enhanced detection methods, increased awareness, and lifestyle changes.









